Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2024; 33(1): 25-35
Published online February 29, 2024
https://doi.org/10.5607/en23030
© The Korean Society for Brain and Neural Sciences
Intranasal Administration of BDNF Improves Recovery and Promotes Neural Plasticity in a Neonatal Mouse Model of Hypoxic Ischemia
Serena-Kaye Sims1,2, Madelynne Saddow1,2, Lilly McGonegal1,2 and Catrina Sims-Robinson1,3*
1Department of Neurology, Medical University of South Carolina, Charleston, SC 29425,
2Department of Biology, College of Charleston, Charleston, SC 29424,
3Ralph H Johnson Veterans Affairs Medical Center, Charleston, SC 29401, USA
Correspondence to: *To whom correspondence should be addressed.
TEL: 1 (843)-792-0851, FAX: 1 (843) 876-1220
e-mail: robinsoc@musc.edu
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The benefit of intranasal brain derived neurotrophic factor (BDNF) treatment on cognitive function in a neonatal postnatal day 7 (P7) mouse model of hypoxic ischemia (HI) was explored. Intranasal delivery is attractive in that it can promote widespread distribution of BDNF within both the brain and spinal cord. In this study we evaluated the effectiveness of intranasal BDNF to improve cognitive recovery following HI. HI is induced via ligation of the right carotid artery followed by a 45-minute exposure to an 8% oxygen/ 92% nitrogen mixture in an enclosed chamber. Male and female pups were subjected to a 2-hour hypothermia in a temperature-controlled chamber as a standard of care. A solution of saline (control) or recombinant human BDNF (Harlan Laboratories) was administered with a Gilson pipette at the same time each day for 7 days into each nasal cavity in awake mice beginning 24 hours after HI. We evaluated cognitive recovery using the novel object recognition (NOR) and western analysis to analyze neuro-markers and brain health such as synaptophysin and microtubule associated protein -2 (MAP2). The objective of this study was to evaluate the role and therapeutic potential of BDNF in neonatal HI recovery. Our results indicate that intranasal BDNF delivered within 24 hours after HI improved object discrimination at both 28 and 42 days after HI. Our results also demonstrate increased synaptophysin and MAP2 at day 42 in HI animals that received intranasal BDNF treatment compared to HI animals that were administered saline.
Graphical Abstract
Keywords: Drug administration, Neural plasticity, Neurodevelopment
INTRODUCTION
Neonatal hypoxic ischemia (HI), caused through a lack of oxygen flow to the brain, can occur as frequently as 1 in every 2,500 live births. HI infants have an increased risk of experiencing a hypoxic ischemic event and having subsequent lifelong neurological disabilities including cerebral palsy, epilepsy, behavioral disorders, and neuropsychological impairments.
There is a need to find safe and effective treatments for HI that improves functional outcomes into adulthood. Research over the past decade has focused on potential prognostic biomarkers of HI recovery. One of these potential biomarkers and mediators of HI recovery is believed to be brain derived neurotrophic factor (BDNF). Preclinical and clinical research over the last decade has identified a role for BDNF in brain plasticity within the intact brain and following central nervous system damage [1-5].
Mature BDNF is first synthesized as the precursor proBDNF and then cleaves to become the mature form of BDNF. Un-cleaved proBDNF can exert its apoptotic effects through its binding to the p75-NTR receptor. While little is known about the impact of exogenous administration of BDNF in neonates, clinical studies in adults and preclinical studies in animals have demonstrated that pharmacologically enhancing BDNF intracerebrally or endogenously enhancing BDNF through exercise enhances synaptic plasticity, reduces infarct size, and aids in motor recovery, learning and memory [6, 7]. Research has extensively documented that BDNF binds to tropomyosin kinase-B (TrkB) receptors at a higher affinity than other neurotrophic receptors to exert its neuroprotective and survival actions, increasing the chances of functional recovery after HI [8]. A small clinical study demonstrated that BDNF levels were lower in preterm infants with factors known to be associated with deleterious developmental outcomes [9]. The exogenous administration of BDNF could be a valuable therapeutic approach for neonatal HI [10, 11]. However, while neurotrophic factors offer neuroprotection, the large neurotrophic protein molecules do not efficiently cross the blood brain barrier. Strategies such as intrahippocampal injection or viral vector upregulation have been utilized in preclinical animal models [12, 13] however, they lack practical clinical application in neonates. On the other hand, intranasal delivery is attractive in that it is non-invasive and bypasses the blood brain barrier with minimal side effects [14]. Intranasal delivery of pain medications such fentanyl and midazolam are already used in infants in which oral delivery is not possible [15-17].
HI is a devastating occurrence that either leads to death or adverse functional outcomes. Intranasal administration of BDNF through the nasal cavity is a noninvasive mode of administration that bypasses the blood brain barrier allowing direct access to the brain [18]. The objective of this study was to determine the efficacy of intranasal BDNF administration on functional recovery in a preclinical neonatal HI model. As HI recovery is further enhanced through dendritic outgrowth and strengthened synapses, we also aimed to determine whether intranasal BDNF enhances neural plasticity and thus functional recovery after HI.
MATERIALS AND METHODS
Animals
Animals were housed in a pathogen-free, temperature-controlled environment, and placed on a 12 hour light and 12 hour dark cycle. All procedures were conducted during the light cycle in accordance with the Guide for the Care and Use of Laboratory Animals adopted by the National Institutes of Health and approved by the Medical University of South Carolina’s Animal Care and Use Committee. Animal Welfare Assurance Numbers on file with the NIH Office of Laboratory Animal Welfare (OLAW) are A3428-01 (Medical University of South Carolina). The university is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC, Intl). The experiments were reported based on ARRIVE guidelines.
Time pregnant dams (C57BL6, B6, mice) were ordered (Jackson laboratories, Bar Harbor, ME) and arrived between gestational day 11~15. Pups were housed with and fed by their dams until post-natal day (PND) 7 (Experiment 1) or PND21 (Experiment 2). For experiment 2, pups were weaned and separated from the dam and grouped and housed according to sex. Upon weaning at PND 21, animals were provided a Harlan Diet and given access to water
Experiment 1: Determing the optimal intranasal BDNF concentration
A separate cohort of PND 7 mice was used to determine the optimal concentration for intranasal BDNF administration via the administration of various concentrations of recombinant human BDNF (5, 10, and 15 µg; Harlan Laboratories) using a Gilson pipette (n=5 per group). Saline was also administered intranasally as a control. Animals were euthanized with isoflurane after one hour. Cortex and hippocampal brain sections were dissected and flash frozen with liquid nitrogen and stored in a -80°C freezer for storage. Samples were later thawed and homogenized in tissue protein extraction reagent (TPER; #78510, Thermo Fisher; Rockford, IL) supplemented with a protease inhibitor cocktail tablet (EDTA; #11836170001; Sigma-Aldrich; St. Louis, MO) according to the manufacturer’s instructions. Samples were then processed later for Western analysis.
Experiment 2: Examing the impact of intransal BDNF on cognitive recovery and neural plasticity
Hypoxic ischemic injury model
The modified Rice-Vannucci model was utilized to induce hypoxic injury at PND 7. With this method, the right carotid artery was ligated. Following a one hour feeding period with maternal dam, pups were subjected to a 45-minute exposure to an 8% oxygen/ 92% nitrogen gas mixture in an enclosed chamber [19-21]. All pups undergoing HI were subjected to 2 hour hypothermia in a temperature-controlled chamber set at 30 degrees Celsius as a standard of care, which was followed by a warming period of 30 minutes. Following hypothermia and warming, mice were returned to the dam in the home cage.
Intranasal BDNF administration
15 µg of recombinant human BDNF (R&D Systems #248-BDB, Minneapolis, MN) was administered with a Gilson pipette for 7 days at PND 8 through PND 15. The BDNF administered has a predicted molecular mass of 13.5 kDA, which is associated with mature BDNF, with a 100% sequence homology with mouse and rat. BDNF was reconstituted with saline upon arrival, distributed into 10 µl aliquots, and frozen at -20°C to avoid multiple freeze thaw cycles. Administration of intranasal BDNF or saline (control) was implemented at 24 post-HI. Intranasal saline and BDNF were administered in 2 µl increments, alternating between each nostril, with 2-minute increments between each nasal administration for a maximum dose volume of 8 µl. Pups received BDNF intranasally while awake to avoid the confounding neuroprotective effect of isoflurane [14].
Cognitive recovery assessments
Cognitive function was assessed on PND 28 and day PND 42. Working memory was evaluated using the novel object recognition task (NOR) as previously described in Sims-Robinson 2016 [22]. For the NOR task, there is an exploration and testing phase. During the exploration phase, mice were placed in the center of an arena where they are allowed to explore two similar objects for 10 minutes. Mice were then placed back in their home cage for one hour and the arena is cleaned with Rescue fragrance free disinfectant and deodorizer (#002240) from Virox Technologies Inc. (ON, Canada). The objects were placed back in the arena with one of the objects being the same and one of the previous objects being replaced by a new (novel) object [23]. After 10 minutes of exploration, the mice were placed back into their home cage and the arena is cleaned again. Microsoft LifeCam HD cameras were used to record behavioral tasks and the behaviors were tracked with the Panlab SMART 3.0 software.
Tissue preparation and processing
At PND 43, mice were euthanized and brains were perfused with 4% PFA, and frozen on dry ice. All tissue was stored at -80 degrees Celsius. Ipsilateral and contralateral cortical and hippocampal tissue were dissected. Tissue was immediately flash frozen with liquid nitrogen and stored in a -80°C freezer for storage. Samples were then processed later for western blot analysis using our previously published protocol [22]. In preparation for western analysis, tissue was briefly homegized. Tissue protein extraction reagent (TPER #7850, Thermo Fisher; Rockford, IL) and protease inhibitor cocktail tablet (EDTA #11836170001; Sigma-Aldrich; St. Louis, MO) was utilized for tissue homogenization. Tissue was then centrifuged for 5 minutes at 14,000 RPM. The supernanatent was then used for a protein assay.
Protein assay and Western analysis
To determine the amount of protein within samples, Bovine Serum Albumin (BSA #A2153; Sigma-Adrich, St. Louis, MO) was utilized to generate a standard curve. The standards and samples were mixed with Pierce Protein Assay Reagent (322660; Thermo Fisher; Rockford, IL). The protein concentration of the samples were interpolated from the BSA standard curve (0, 100, 250, 500, 750, 1000, 2000 μg), and lysates with 7 μg of protein were prepared for assessment of BDNF, proBDNF, MAP2, and Synaptophysin western analysis.
Western immunoblotting was performed per our previously published protocol. Relative protein levels of BDNF (14 kDA), proBDNF (32 kDA), MAP2 (280 kDa) and Synaptophysin (38 kDa) was measured through this protocol. Briefly, previously prepared lysates were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. Samples were blocked and primed in either 5% Milk of 5% BSA in TBST. Western analysis was performed in sections of mouse cortex and hippocampus using BDNF Polyclonal Antibody (Product #PA5-85730, Thermo Fisher Scientific, Waltham, MA), Anti-MAP2 antibody (1:1000; Product #MAB3 428, Sigma Aldrich, St. Louis, MO) and synaptophysin antibody (1:1000; Product #4329, Cell Signaling, Danvers, MA). The appropriate horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology) were used at a 1:2000 dilution. Band quanitification was visualized using Clarity Western ECL Substrate (Bio-Rad Laboratory, Hercules, CA), with images captured using ChemiDoc™ Imaging System, analyzed by Image Lab software (Bio-Rad Laboratory), and normalized to total lane protein concentrations.
Study design
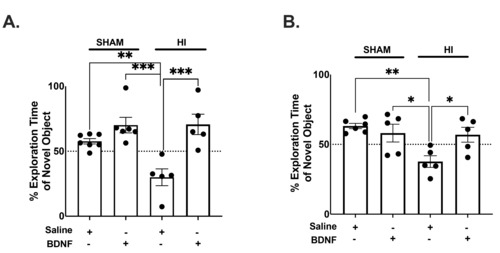
The overall study design for Experiment 2 is depicted in Fig. 1. PND7 mice (n=22) were separated into 4 groups of mice: 1) Sham with intranasal saline at 24 hr (n=6), 2) Sham with intranasal BDNF at 24 hr (n=6), 3) HI with intranasal BDNF at 24 hr/ hypothermia (n=5), 4) HI with intranasal saline at 24 hr/hypothermia (n=5). Sham operated animals did not undergo HI.
Rigor and statistical analysis
The laboratory adheres to the principles of Good Laboratory Practice (GLP). Data analyses were performed with Prism v9 GraphPad Software, Inc (La Jolla, CA) and SPSS (IBM SPSS Statistics Data Editor Version 18.2). Differences between groups for the western analysis for mature BDNF, proBDNF, MAP2 and Synaptophysin were analyzed using the analysis of variance (ANOVA) model. Post hoc comparisons were made using a Tukey multiple compairsons test. A linear mixed model was utilized to analyze both NOR time points. All data was reported as mean±standard deviation (SD) with significance p<0.05. To ensure scientific rigor and unbiased results: 1) all animals were randomly assigned to treatment groups. 2) All behavioral assessments were recorded and saved using a video camera. 3) All behavioral acquisitions were conducted by an experimenter who was blinded to the experimental conditions. 4) Analysis was conducted by a well-trained observer (different from experimenter) who was also blinded to the experimental conditions, using automated tracking software (PanLab SMART).
RESULTS
Intranasal BDNF increases mature and proBDNF in neonates
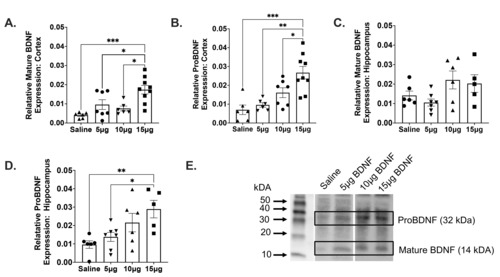
A one-way ANOVA was performed to compare the effect of intranasal BDNF on both mature and proBDNF. There was a statistically significant difference among groups analyzed for both mature BDNF (F(3,24)=8.439, p=.0005) and proBDNF (F(3,24)=10.76, p=.0001) within the cortex. Tukey’s post-hoc analysis revealed greater concentrations of intranasal BDNF resulted in greater BDNF protein levels (Saline vs. 15 µg, p=.0393), (5 µg vs. 15 µg, p=.0102), (10 µg vs. 15 µg, p=.0005) (Fig. 2A) and proBDNF (Saline vs. 15 µg, p=.0411), (5 µg vs. 15 µg, p=.0010), (10 µg vs. 15 µg, p=.0002) (Fig. 2B). Results also revealed that there were no significant differences in relative mature BDNF levels in the neonatal hippocampus after intranasal administration of various concentrations of BDNF (Fig. 2C). Greater relative levels of BDNF did result in greater proBDNF protein levels in the hippocampus. A one way ANOVA revealed that there was also a statistically significant difference between groups analyzed for proBDNF F(3,20)=5.102, p=.0088) within the hippocampus. Post-hoc analysis using Tukey’s Honestly Significant Difference (HSD) test revealed significant differences in proBDNF expression within the hippocampus between saline and 15 µg of BDNF (p=.0094) and between 5 µg and 15 µg of BDNF (p=.0409) (Fig. 2D).
Intranasal BDNF improves cognitive recovery in neonatal mice with HI
To determine the long-term cognitive impact in HI neonates, novel object recognition (NOR) was evaluated at both PND 28 and 42. A Linear Mixed Model was utilized to determine the impact of treatment on time spent exploring the novel object, indicative of normal memory. There is an overall group interaction (F(3,16)=12.18, p=.0002). HI+Saline animals also explored the novel object significantly more at both PND 28 (p=.0099) and PND 42 (p=.0042), indicating. HI causes memory impairment (Fig. 3A, B). NOR post-hoc analysis more at day 28 demonstrated that BDNF may rescue these HI induced cognitive deficits. HI+BDNF animals explored the novel object more than the familiar object when compared to HI+Saline animals (p=.0006) (Fig. 3A). At PND 42, NOR results demonstrated that HI+BDNF animals still interacted longer with the novel object compared to HI+Saline animals (p=.0426) (Fig. 3B).
Intranasal BDNF promotes increases in markers of neural plasticity in neonatal mice with HI
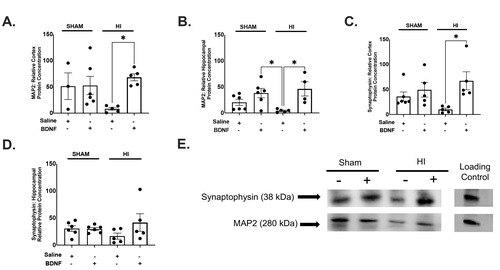
To evaluate the potential impact of HI and BDNF treatment on neural plasticity, we compared the effects of intranasal BDNF on MAP2 and Synaptophysin in both the cortex the hippocampus. A one-way ANOVA was performed to compare the effect of intranasal BDNF on MAP2 within the cortex and hippocampus. A one-way ANOVA revealed that there was a statistically significant difference among groups analyzed in the cortex (F(3,18)=3.864, p=.0270) and hippocampus (F(3,17)=5.017, p=.0113) (Fig. 4A, B). Tukey’s HSD post-hoc analysis revealed increased MAP2 in HI animals treated with BDNF compared HI animals treated with saline in the cortex. Analysis demonstrated an increase in microtubule associated protein-2 in HI+BDNF animals compared to both HI+saline (p=.0218) (Fig. 4A). Hippocampal MAP2 post-hoc analysis also revealed an increase in MAP2 in HI+BDNF animals compared to HI+saline (p=.0160). Western analysis also revealed a decrease in MAP2 in HI+Saline animals compared to SHAM+BDNF animals (p=.0332) (Fig. 4B).
BDNF downstream activation through interaction with the TrkB receptor promotes axon growth and maintenance as well as synaptic differentiation. Synaptophysin is an integral membrane glycoprotein that occurs in presynaptic vesicles of neurons. A one-way ANOVA was performed to compare the effect of intranasal BDNF on synaptophysin within the cortex and hippocampus. A one-way ANOVA revealed that there was a statistically significant difference between groups analyzed in the cortex (F(3,17)=3.720, p=.0319) but not within the hippocampus (F(3,18)=1.371, p=.2835) (Fig. 4C, D). Post-hoc analysis with Tukey’s HSD test revealed greater protein concentration in HI+BDNF compared with HI+Saline (p=.0228) within the cortex (Fig. 4C). Hippocampal Synaptophysin western analysis demonstrated no significant differences between treatment groups and synaptophysin within the hippocampus (Fig. 4D).
The relationship between cognitive recovery and neural plasticity
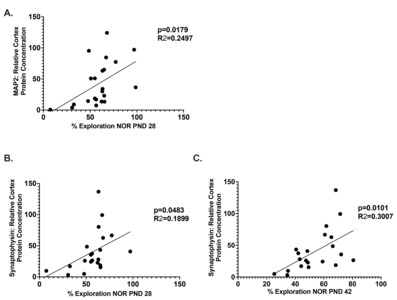
The causal relationship between neural recovery and functional recovery is well established in the literature. Linear regression analysis revealed a possible relationship between time spent with the novel object on the NOR assessment and between MAP2 and Synaptophysin. Analysis demonstrated a positive correlation between MAP2 and time spent with the novel object at PND28 (p=0.0179, R=0.2497) (Fig. 5A). There was also a positive correlation between Synaptophysin and time spent with the novel object on both PND 28 (p=0.042, R=0.1899) (Fig. 5B) and PND 42 (p=0.0101, R=0.3007) (Fig. 5C).
DISCUSSION
Neonatal HI is a devastating occurrence that either leads to death or adverse cognitive outcomes. As there are few therapeutic interventions for HI, it is important to identify an effective treatment paradigm that would improve the cognitive dysfunction that occurs as a result of HI. Hence, the goal for this study was to determine the efficacy of intranasal BDNF administration on functional recovery in a preclinical neonatal HI model in which clinical care is limited to hypothermia.
Our preliminary experiments began with determining the optimal concentration of BDNF for treatment by assessing BDNF levels in target regions, namely the cortex and hippocampus. The cortex and hippocampus are the most vulnerable and present the most severe cognitive impairment in MRI studies involving HI [24-26]. As there are no previous studies involving the intranasal administration of BDNF and other neurotrophins, we tested various concentrations between 10 and 80 µg. In a study involving adult rats, 70 µg of BDNF was intranasally administered to determine the concentration of BDNF in various brain regions one to three hours after initial administration [14]. A concentration of 40 µg/kg of BDNF was intranasally delivered in an adult mouse in a depression model mouse [27]. In an additional study, a 42 pmol dose of BDNF was found to be effective in improving cognitive performance in an Alzheimer’s Disease mouse model [28].
Given that there are variabilities between neonates and adults and TrkB, activation of the potassium chloride co-transporter (KCC2) has been linked to increase chances of seizures after HI [29, 30], we tested significantly lower concentrations of BDNF in a subset of neonatal mice. It was important to administer smaller concentrations of BDNF to prevent over activation of the TrkB receptor. Our data reveals that 15 µg of BDNF resulted in significant increases in both mature and proBDNF within the cortex. Intranasal administration of 15 µg of BDNF resulted in increased proBDNF within the hippocampus. As proBDNF is a known precursor for mature BDNF, our data reveals that intranasal BDNF may also promote the production of more BDNF. As exogenous BDNF binds to TrkB receptors, pro forms of BDNF are synthesized in the Golgi to increase levels of mature BDNF [31]. While it is possible that intranasal delivery of BDNF resulted in activity-dependent production of proBDNF. It is also possible that the mature BDNF protein quantification in Experiment 1, measures both activity-dependent production of BDNF and the BDNF that was intranasally delivered. This may because the Recombinant Human BDNF (13.5 kDa) that was intranasally administered has a 100% sequence homology with mouse and rat and both the mature BDNF and proBDNF antibodies have reactivity with human, rat and mouse. While it is a possibility that western immunoblotting for mature BDNF measures the administered BDNF, it does not explain the increases in proBDNF. It is a possibility that intranasal delivery of BDNF results in activity- dependent increases in proBDNF and thus BDNF. Recombinant mature BDNF may lead to an increase in gene expression, thus increasing the mature BDNF precursor, proBDNF. So, while measurements of mature BDNF may be partially the result of administered recombinant BDNF, due to the size difference of proBDNF, this is not the case. Due to the short half-life of BDNF, and the speed at which intranasal delivery allows for permeability into the brain, it is likely to result in rapid BDNF expression changes that are indicated by our western analysis quantification. Several publications have indicated that stress and exogenous BDNF can result in changes in BDNF expression in under 60 seconds [32-34].
In experiment 2, the impact of 15 µg of intranasal BDNF administration over the course of 7 days on cognition was assessed in HI animals. Several clinical and preclinical studies have demonstrated that voluntary physical exercise promotes BDNF expression, increases hippocampal BDNF levels, and improves neurological outcomes after experimental stroke [5]. Previous studies established that cognitive rescue after stroke and other disorders are BDNF-dependent. Studies involving infusions, intraperitoneal, or mini-pump administration of BDNF after stroke demonstrates that animals treated with BDNF had fewer working and reference memory and greater cognitive recovery compared to saline animals [35]. Similiarily, our data reveals that intranasal BDNF treatment improved cognitive outcomes in HI aminals compared to HI animals treated with intranasal saline.
To determine whether improvements in cognitive recovery were related to increases in neural plasticity. Our study replicated experiments performed in adult rodents that BDNF administration has the potential to increase dendritic and synaptic plasticity [36-38]. In this study, we measured dendritic spine density using Microtubule-associated protein 2 (MAP2) as it is the predominant cytoskeletal regulator within neuronal dendrites. Studies have implicated the involvement in BDNF in the development of spines during early development and the deficits in the maturation and pruning of spines in cases of Autism, cerebral palsy, and other neurocognitive disorders [39-43].
Synaptophysin is a reliable indicator of synaptic plasticity, and has previously been demonstrated to correlate well with the loss of cognitive function in mouse models. BDNF is believed to induce rapid effects on synaptic transmission by inducing the release of glutamate and GABA through its binding with TrkB to phosphorylate synapsin (a protein important for synapse stabilization) [44, 45]. The phosphorylation of synapsin also results in increased numbers of docked vesicles at the active zone of synapses [46]. Our results demonstrate that intranasal BDNF treatment results in higher cortical levels of synaptophysin and MAP2, which suggests greater neuroplasticity is associated with BDNF treatment. We did not see these same significant increases in synaptophysin levels within the cortex in HI+BDNF treated animals. There may be a few reasons for this. While intranasal delivery does project to several brain areas such as the thalamus, cerebral cortex, optic nerve, trigeminal nerve, and many other areas, it is possible that intranasal delivery of larger proteins such as BDNF do not permeate subcortical regions to the same degree as other areas. Therapeutics either travel by the trigeminal nerve to reach midbrain and hindbrain vasodilatory areas or travel through the olfactory nerve through the nasal mucosa to penetrate the brain [47, 48]. A previous study was performed to determine which areas exhibited the highest concentrations of proteins after intranasal administration of various neurotransmitters [14]. The highest concentrations of BDNF resulted in greater penetration of the trigeminal nerve and olfactory bulb, after 25~60 minutes after intranasal delivery. While the hippocampus and frontal cortex had comparable concentration levels after 60 minutes of delivery, there were slightly higher levels of BDNF within the cortex at 25 minutes compared to the hippocampus [14]. These studies may further elucidate why, in our preliminary experiment where we tested various concentrations of BDNF, we do not see significant differences within the hippocampus between animal groups measured for mature BDNF. This may also further explain why we do not see significant differences along with lower concentrations of synaptophysin within the hippocampus and even slightly smaller concentrations of MAP2 within the hippocampus of animals tested for MAP2.
While the role of neural recovery on functional recovery is well established, to our knowledge, we are the first to demonstrate in a neonatal mouse model of HI, that intranasal BDNF results in improved cognitive and neural recovery. Our data also demonstrates, through correlation analysis, a significant positive relationship between markers of neural plasticity and cognitive recovery.
CONCLUSION
This study evaluated the role and therapeutic potential of BDNF in stroke recovery in a preclinical neonatal stroke model. We and other studies have demonstrated the ability of BDNF to influence functional recovery and its neural properties of recovery. Intranasal administration of BDNF is a novel treatment and non-invasive delivery system that has great therapeutic potential in vulnerable infants, for whom limited therapies exist.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (NIH): NINDS Blueprint DSPAN K00 NS105220 to S-KS, NINDS R01 NS099595 to CS-R, NHLBI R25 GM072643 to CS-R, and NIGMS P20 GM109040 to S-KS and CS-R, the Department of Veterans Affairs I01 BX005666 to CS-R, and the Alzheimer’s Association AARGD-23-970621 to CS-R.
Figures
References
- Houlton J, Zhou LYY, Barwick D, Gowing EK, Clarkson AN (2019) Stroke induces a BDNF-dependent improvement in cognitive flexibility in aged mice. Neural Plast 2019:1460890
- Wurzelmann M, Romeika J, Sun D (2017) Therapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury. Neural Regen Res 12:7-12
- Korley FK, Diaz-Arrastia R, Wu AH, Yue JK, Manley GT, Sair HI, Van Eyk J, Everett AD, Okonkwo DO, Valadka AB, Gordon WA, Maas AI, Mukherjee P, Yuh EL, Lingsma HF, Puccio AM, Schnyer DM; TRACK-TBI investigators (2016) Circulating brain-derived neurotrophic factor has diagnostic and prognostic value in traumatic brain injury. J Neurotrauma 33:215-225
- Liu W, Wang X, O'Connor M, Wang G, Han F (2020) Brain-derived neurotrophic factor and its potential therapeutic role in stroke comorbidities. Neural Plast 2020:1969482
- Alcantara CC, García-Salazar LF, Silva-Couto MA, Santos GL, Reisman DS, Russo TL (2018) Post-stroke BDNF concentration changes following physical exercise: a systematic review. Front Neurol 9:637
- Miranda M, Morici JF, Zanoni MB, Bekinschtein P (2019) Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci 13:363
- Ploughman M, Windle V, MacLellan CL, White N, Doré JJ, Corbett D (2009) Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke 40:1490-1495
- Sadick MD, Galloway A, Shelton D, Hale V, Weck S, Anicetti V, Wong WL (1997) Analysis of neurotrophin/receptor interactions with a gD-flag-modified quantitative kinase receptor activation (gD.KIRA) enzyme-linked immunosorbent assay. Exp Cell Res 234:354-361
- Rao R, Mashburn CB, Mao J, Wadhwa N, Smith GM, Desai NS (2009) Brain-derived neurotrophic factor in infants <32 weeks gestational age: correlation with antenatal factors and postnatal outcomes. Pediatr Res 65:548-552
- Simmons DA, Rex CS, Palmer L, Pandyarajan V, Fedulov V, Gall CM, Lynch G (2009) Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice. Proc Natl Acad Sci U S A 106:4906-4911
- Clarkson AN, Parker K, Nilsson M, Walker FR, Gowing EK (2015) Combined ampakine and BDNF treatments enhance poststroke functional recovery in aged mice via AKT-CREB signaling. J Cereb Blood Flow Metab 35:1272-1279
- Knüsel B, Beck KD, Winslow JW, Rosenthal A, Burton LE, Widmer HR, Nikolics K, Hefti F (1992) Brain-derived neurotrophic factor administration protects basal forebrain cholinergic but not nigral dopaminergic neurons from degenerative changes after axotomy in the adult rat brain. J Neurosci 12:4391-4402
- Blits B, Oudega M, Boer GJ, Bartlett Bunge M, Verhaagen J (2003) Adeno-associated viral vector-mediated neurotrophin gene transfer in the injured adult rat spinal cord improves hind-limb function. Neuroscience 118:271-281. https://doi.org/10.1016/s0306-4522(02)00970-3
- Alcalá-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH 2nd, McLoon LK (2010) Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J Drug Target 18:179-190
- Ku MS (2018) Neonatal phototherapy: a novel therapy to prevent allergic skin disease for at least 5 years. Neonatology 114:235-241
- Harlos MS, Stenekes S, Lambert D, Hohl C, Chochinov HM (2013) Intranasal fentanyl in the palliative care of newborns and infants. J Pain Symptom Manage 46:265-274
- Nathan CO, Seid AB (1997) Neonatal rhinitis. Int J Pediatr Otorhinolaryngol 39:59-65
- Hanson LR, Frey WH 2nd (2008) Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci 9(Suppl 3):S5
- Jatana M, Singh I, Singh AK, Jenkins D (2006) Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res 59:684-689
- Vannucci SJ, Hagberg H (2004) Hypoxia-ischemia in the immature brain. J Exp Biol 207(Pt 18):3149-3154
- Gail MW, Sims-Robinson C, Boger H, Ergul A, Mukherjee R, Jenkins DD, George MS (2023) Transcutaneous auricular vagus nerve stimulation (taVNS) decreases heart rate acutely in neonatal rats. Brain Stimul 16:1240-1242
- Sims-Robinson C, Bakeman A, Rosko A, Glasser R, Feldman EL (2016) The role of oxidized cholesterol in diabetes-induced lysosomal dysfunction in the brain. Mol Neurobiol 53:2287-2296
- Barker GR, Warburton EC (2011) When is the hippocampus involved in recognition memory?. J Neurosci 31:10721-10731
- Logitharajah P, Rutherford MA, Cowan FM (2009) Hypoxic-ischemic encephalopathy in preterm infants: antecedent factors, brain imaging, and outcome. Pediatr Res 66:222-229
- Zhang H, Roman RJ, Fan F (2022) Hippocampus is more susceptible to hypoxic injury: has the Rosetta Stone of regional variation in neurovascular coupling been deciphered?. Geroscience 44:127-130
- Baburamani AA, Ek CJ, Walker DW, Castillo-Melendez M (2012) Vulnerability of the developing brain to hypoxic-ischemic damage: contribution of the cerebral vasculature to injury and repair?. Front Physiol 3:424
- Li XL, Liu H, Liu SH, Cheng Y, Xie GJ (2022) Intranasal administration of brain-derived neurotrophic factor rescues depressive-like phenotypes in chronic unpredictable mild stress mice. Neuropsychiatr Dis Treat 18:1885-1894
- Braschi C, Capsoni S, Narducci R, Poli A, Sansevero G, Brandi R, Maffei L, Cattaneo A, Berardi N (2021) Intranasal delivery of BDNF rescues memory deficits in AD11 mice and reduces brain microgliosis. Aging Clin Exp Res 33:1223-1238
- Martinet LE, Ahmed OJ, Lepage KQ, Cash SS, Kramer MA (2015) Slow spatial recruitment of neocortex during secondarily generalized seizures and its relation to surgical outcome. J Neurosci 35:9477-9490
- Kipnis PA, Sullivan BJ, Carter BM, Kadam SD (2020) TrkB agonists prevent postischemic emergence of refractory neonatal seizures in mice. JCI Insight 5:e136007
- Pang PT, Lu B (2004) Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev 3:407-430
- Marmigère F, Givalois L, Rage F, Arancibia S, Tapia-Arancibia L (2003) Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus 13:646-655
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME (1998) Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20:709-726
- Esvald EE, Tuvikene J, Moistus A, Rannaste K, Kõomägi S, Timmusk T (2022) Differential regulation of the BDNF gene in cortical and hippocampal neurons. J Neurosci 42:9110-9128
- Kiprianova I, Sandkühler J, Schwab S, Hoyer S, Spranger M (1999) Brain-derived neurotrophic factor improves long-term potentiation and cognitive functions after transient forebrain ischemia in the rat. Exp Neurol 159:511-519
- Qiao H, An SC, Xu C, Ma XM (2017) Role of proBDNF and BDNF in dendritic spine plasticity and depressive-like behaviors induced by an animal model of depression. Brain Res 1663:29-37
- Thacker JS, Mielke JG (2022) The combined effects of corticosterone and brain-derived neurotrophic factor on plasticity-related receptor phosphorylation and expression at the synaptic surface in male Sprague-Dawley rats. Horm Behav 145:105233
- Wang J, Cai Y, Sun J, Feng H, Zhu X, Chen Q, Gao F, Ni Q, Mao L, Yang M, Sun B (2023) Administration of intramuscular AAV-BDNF and intranasal AAV-TrkB promotes neurological recovery via enhancing corticospinal synaptic connections in stroke rats. Exp Neurol 359:114236
- Chapleau CA, Larimore JL, Theibert A, Pozzo-Miller L (2009) Modulation of dendritic spine development and plasticity by BDNF and vesicular trafficking: fundamental roles in neurodevelopmental disorders associated with mental retardation and autism. J Neurodev Disord 1:185-196
- Barbosa AG, Pratesi R, Paz GSC, Dos Santos MAAL, Uenishi RH, Nakano EY, Gandolfi L, Pratesi CB (2020) Assessment of BDNF serum levels as a diagnostic marker in children with autism spectrum disorder. Sci Rep 10:17348
- von Bohlen Und Halbach O, von Bohlen Und Halbach V (2018) BDNF effects on dendritic spine morphology and hippocampal function. Cell Tissue Res 373:729-741
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS (2004) BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7:48-55
- Perovic M, Tesic V, Mladenovic Djordjevic A, Smiljanic K, Loncarevic-Vasiljkovic N, Ruzdijic S, Kanazir S (2013) BDNF transcripts, proBDNF and proNGF, in the cortex and hippocampus throughout the life span of the rat. Age (Dordr) 35:2057-2070
- Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czernik AJ (1996) Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci U S A 93:3679-3683
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ (2004) Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J Neurosci 24:522-530
- Tyler WJ, Pozzo-Miller LD (2001) BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci 21:4249-4258
- Johnson NJ, Hanson LR, Frey WH (2010) Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol Pharm 7:884-893
- Lochhead JJ, Kellohen KL, Ronaldson PT, Davis TP (2019) Distribution of insulin in trigeminal nerve and brain after intranasal administration. Sci Rep 9:2621